The CDMO Expert
in Cell Therapy
Products
Cell-based therapies are not conventional Biologics,
they are living drugs…
…requiring cutting-edge expertise in Cell Culture.
Clinical Doses
manufactured in our state-of-the-art GMP facilities

Cell types
T cells, NK cells, T regs, MSCs, iPS cells, Macrophages, immortalized cell lines,…

Development Programs
Process Development, Optimization, Scale up.
Analytical & Potency test development.

ATMP Developers
TRUST US
from academic, to Biotech and Pharma companies
How SCIENCE plays out at Cell-Easy ?
By combining solid technological and scientific knowledge in CELL CULTURE with conventional regulatory-controlled CDMO services, Cell-Easy’s multi-disciplinary team support you in transforming your laboratory proof-of-concept into a successful first-in-human clinical trial.
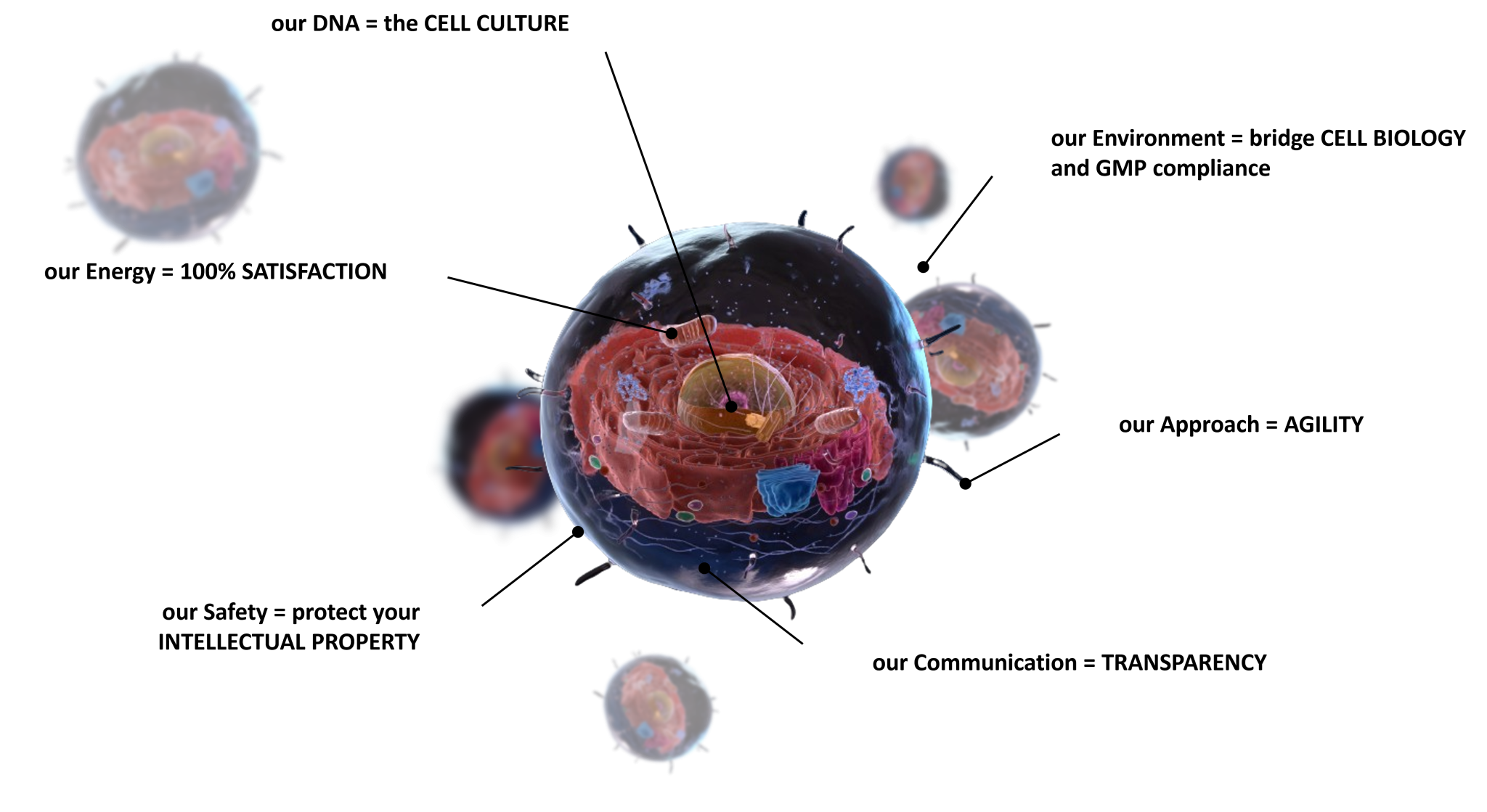
- Teams = PhDs, Engineers, Technicians in Cell Biology
- 70+ Technologies for Process & Analytics Development
- REGULATORY support adapted to cell products
- CMC driven by cell variability
- From concept to First-in-Human and beyond
- A dedicated Project Manager
- Co-define a robust roadmap
- Share Risks & Gaps during weekly meeting
- Avoid Change of Scope
- Accelerate Process Development/Scale-up with our Platforms
- Access expertise on 50+ different technologies
- Pre-established contract with providers
- Beyond agreements (NDA, MSA, QTA) our internal confidential policy protect your industrial secret
- As a fee for service CDMO, no IP is shared
- All documentation/production is Yours
- Respect TIMELINES
- Meet BUDGET
- Bring continous CMC and REGULATORY support
- Your SUCCESS is our goal
Cell-Easy Expands its GMP Cell Therapy Manufacturing Facility
Cell-Easy Expands its GMP Cell Therapy Manufacturing Facility to Support Growing Client Demand. New state-of-the-art class-B cleanrooms, development labs, and logistics upgrades accelerate scalable production of autologous & allogeneic cell therapies in compliance with FDA/EMA standards. Toulouse, France –April 9th – Cell-Easy, a leading European Contract Development and Manufacturing Organization (CDMO) specializing

CTA Approval to initiate ALLOFIST
CHU Toulouse receives CTA Approval to initiate ALLOFIST, a Phase 1/2 Trial based on Cell-Easy’s proprietary adipose-derived MSC in Patients suffering from Crohn Disease. Toulouse, France, January 15, 2025 The long-term partnership between Cell-Easy and Toulouse University Hospital (CHU Toulouse) continues to bear fruits, bringing innovative MSC-based therapies to
Contact
Tel : +33 534 276 550
Address : Cell-Easy SAS, 4 bis avenue Hubert Curien, 31100 Toulouse, France
Mail : info@cell-easy.com



