Discover our Case Studies!
From R&D to GMP: Successful Clinical-Grade Manufacturing of an NK Cell Therapy
- Problem Statement: Transitioning a natural killer (NK) cell therapy from research to GMP-compliant manufacturing requires overcoming major scientific, technical, and regulatory hurdles. Our client, a European biotech, needed to scale up an academic NK cell expansion process into a clinically viable and regulatory-ready cell therapy, without compromising cell functionality, cytotoxicity, or quality standards.
- Our Approach: As a CDMO specilized in Cell Therapy, Cell-Easy developed a tailored, stepwise strategy to tackle each obstacle:
Optimization of NK cell expansion under GMP conditions
GMP-compliant feeder cell process development and scale-up
Implementation of robust cryopreservation and thawing protocols
End-to-end regulatory and CMC support, including the sourcing of GMP-grade cord blood, IMPD preparation and CTA submission
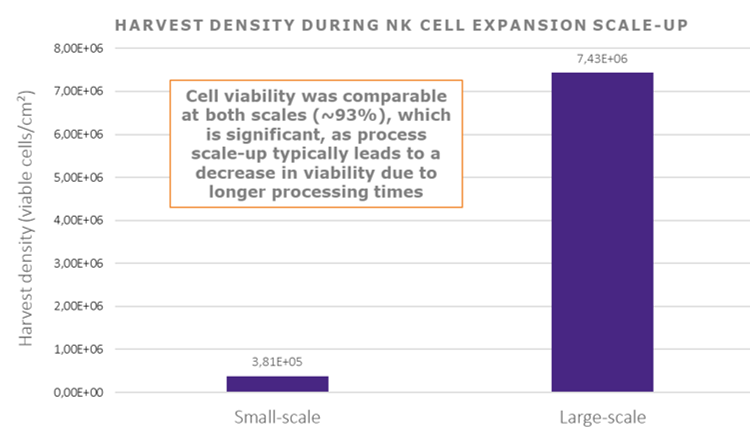
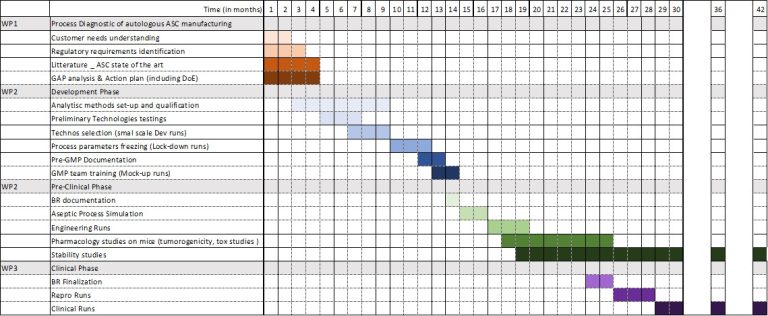
- Outcomes: In 28 months, Cell-Easy successfully translated the R&D process into GMP-compliant manufacturing, releasing one engineering and two clinical batches that met all quality criteria. The work culminated in the approval of the Clinical Trial Application (CTA), enabling the first Phase 1 dosing.
Maximize Working Cell Bank (WCB) Manufacturing Quantity while Controlling Batch Costs
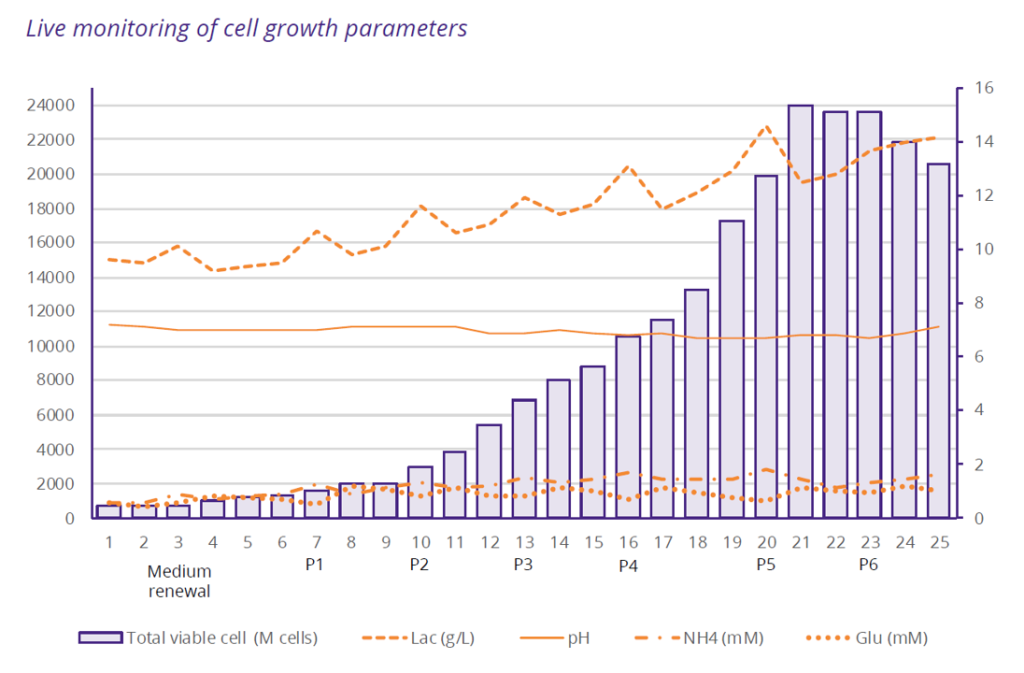
- Problem Statement: To develop a cell therapy product based on NK cells for a PhaseI clinical study, a European biotech company partnered with Cell-Easy to scale up and manufacture a GMP-compliant Working Cell Bank (WCB) from an immortalized feeder cell line. While the biotech had already established the 2 weeks process at lab-scale, the challenge was to scale it up and manufacture within a tight timeframe: 12 months to produce the first GMP batch of 50 billion cells.
- Our Approach: We have adopted a 3-stage strategy:
- Transfer and implementation of analytical methods.
- Control the scale up: Evaluation and testing of culture supports and conditions.
- Implementation in GMP environment.
- Outcomes: Thanks to our Quality by Design approach and the Cell Biology expertise of our multi-disciplinary teams, the WCB manufacturing process was successfully
scaled up — achieving a sixfold increase in cell production compared to the initial process — while maintaining the cell line’s quality attributes, adhering to the overall project timelines. In addition, the control of scale-up, batch-to-batch reproducibility and the increase in cell viability post-thawing have mechanically led to a 3-fold reduction in cost per billion cells manufactured.
An off-the-shelf MSC product derived from lab-scale autologous process
- Problem statement: Drawing on their scientific and medical expertise in the field of autologous MSCs, local researchers, professors and clinicians wanted to offer patients suffering from various autoimmune diseases an affordable and safe treatment.
- Our Approach: In just 30 months, our dedicated teams have accomplished a significant milestone: the successful development, manufacturing, and validation of clinical-grade adipose-derived Mesenchymal Stem Cells (MSCs) based on a large-scale allogeneic process. These cells have received authorization from the French National Agency for Medicinal Products (ANSM) and are being injected in a range of Human clinical studies.
- Outcomes: Cell-Easy is offering R&D and GMP grade adipose-derived MSCs as:
Study model for Research Programs
- Drug Product in various Clinical Trials (systemic sclerosis, Crohn disease, Alzheimer disease, arthritis diseases,…)
- Starting material for the generation of GMP exosomes
- Cargo for various molecules and viruses
Contact
Tel : +33 534 276 550
Address : Cell-Easy SAS, 4 bis avenue Hubert Curien, 31100 Toulouse, France
Mail : info@cell-easy.com