HOW TO ENSURE A SEAMLESS IMPD/IND APPROVAL
The field of cell therapies is poised to redefine the medical landscape, offering hope to patients with previously untreatable diseases. According to BBC research, the global market for cell and gene therapy is expected to grow from $7.2 billion in 2023 and projected to reach $23.3 billion by the end of 2028. However, the journey to market is filled with regulatory challenges, one of the most important being the Investigational Medicinal Product Dossier (IMPD) or Investigational New Drug (IND) application. A well-crafted IMPD/IND is essential for gaining regulatory approval and progressing clinical trials. This document serves as a blueprint, providing regulatory authorities with crucial information about the investigational medicinal product (IMP). By understanding the nuances of the IMPD/IND and navigating the regulatory landscape, companies can accelerate the development of their cell therapies and bring life-changing treatments to patients faster.
THE IMPORTANCE OF IMPD/IND APPROVAL FOR CLINICAL TRIALS
Gaining approval for your IMPD/IND is not just a step in the regulatory process. Failing to meet regulatory requirements can have severe consequences such as delays, increased cost or even the termination of your development journey. The approval of regulatory bodies is essential for progressing your therapy. A well-prepared dossier can significantly influence the trajectory of your clinical trials.
COMMON CHALLENGES IN IMPD/IND SUBMISSIONS FOR ATMPS
- Failure to Meet CMC Requirements: CMC (Chemistry, Manufacturing, and Controls) issues are a major cause of regulatory delays for ATMPs. Ensuring robust, GMP-compliant (The Rules Governing Medicinal Products in the European Union Volume 4 Good Manufacturing Practice) manufacturing processes is critical, as even minor deviations can lead to clinical trial setbacks or failure. Due to the complexity of ATMPs, once the process is defined, it must remain fixed. Any change to the manufacturing process or facilities directly alters the product, as in cell therapy, the process is the product. As a result, additional comparability studies are often required to confirm that safety and efficacy are maintained.
- Inadequate Quality Control Measures: ATMPs require rigorous and comprehensive quality control (QC) measures to ensure the consistency, safety, and efficacy of the product. For ATMPs, the potential risks to patient safety and product quality are significantly higher than for conventional medicinal products. Without robust evidence of quality assurance practices, such as validated testing methods, in-process controls, and stability data, regulatory authorities may delay or reject product submissions.
- Regulatory Misalignment: Different regulatory authorities, such as the FDA and EMA, have their own unique specificities, which can complicate the submission process. Misalignment of regulatory expectations early in the development process can result in costly revisions and delays. Preparing an IND based on an IMPD – and vice versa – is not a copy and paste exercise.
- Incomplete Documentation: Clear, complete, and accurate data on manufacturing processes, quality control measures, and preclinical results are crucial for a successful IMPD/IND submission. Gaps in documentation often lead to requests for additional data from regulatory authorities, extending timelines and inflating costs.
- Evolving Regulatory Landscape: The regulatory landscape for ATMPs is constantly evolving, influenced by scientific advances and shifting regulatory frameworks. Biotech companies must stay up to date with these changes to ensure compliance, often requiring significant resources for regulatory intelligence and strategy.
THE ROLE OF CDMOS IN PREPARING IMPD/IND SUBMISSIONS FOR ATMPS
Partnering with a CDMO can significantly enhance your ability to prepare and submit a successful IMPD/IND for your ATMP. At Cell Easy, our goal is to empower you to meet your specific ATMP project needs through tailored solutions. Our key services include:
- Proactive Regulatory Planning: Cell easy collaborates closely with you, integrating regulatory experts from the outset to ensure a deep understanding of your regulatory requirements. This proactive approach includes mapping out essential documentation, implementing quality control measures, validating methods, and customizing manufacturing processes specific to your ATMP to ensure regulatory compliance and product integrity.
- Tailored Regulatory Strategies: At Cell easy, we understand that each ATMP project is unique, requiring a customized approach to navigate regulatory challenges effectively. This process involves conducting thorough risk assessments, identifying potential roadblocks, and devising targeted mitigation strategies to ensure a smooth approval process.
- Robust Documentation: A well-structured and comprehensive IMPD/IND is essential for a successful regulatory review. Our team, led by Cindy, works diligently with biotech companies to create IMPDs that adhere to regulatory guidelines, providing a clear overview of your ATMP’s quality, safety, and efficacy. Emphasizing concise drafting, rapid responses to regulatory inquiries, and structured information assembly, we ensure that your documentation meets the highest standards throughout the development process.
BEST PRACTICES FOR ENSURING SMOOTH IMPD/IND APPROVAL FOR ATMPS
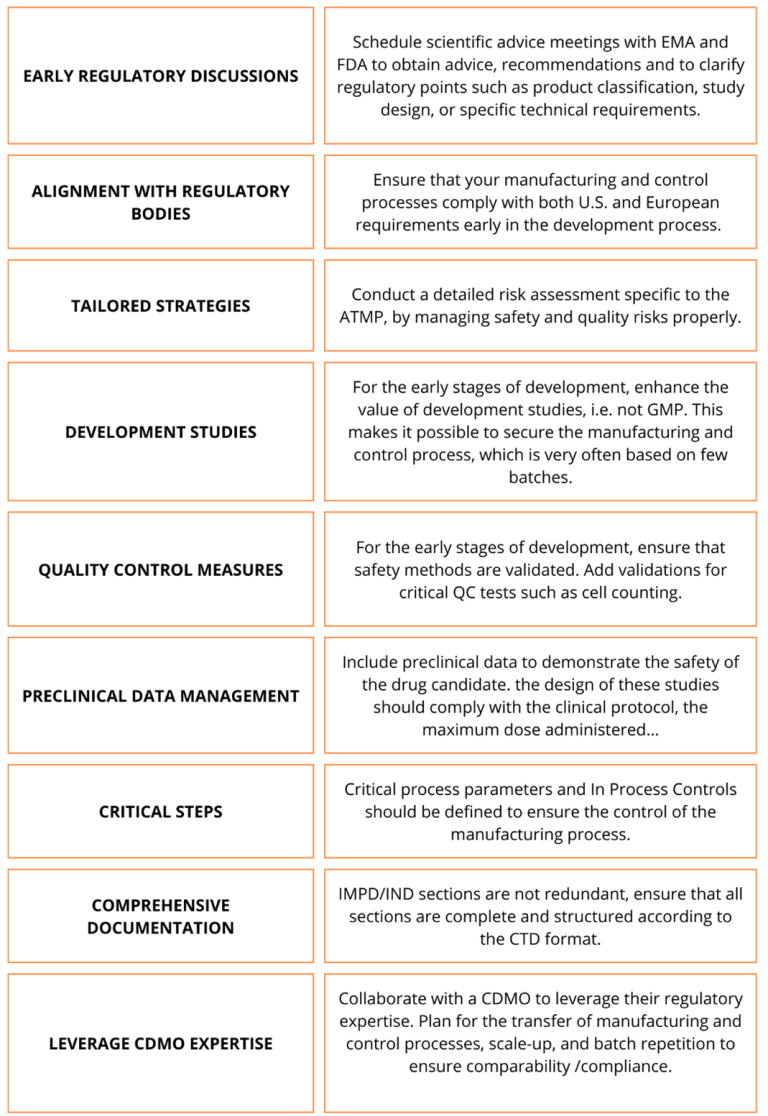
To navigate the complexities of obtaining IMPD/IND approval for ATMPs, biotech companies must adopt proactive strategies to ensure compliance and streamline the approval process. Currently, there is no formal framework for the IMPD/IND for ATMPs. However, it needs to be emphasized that in 2022 EMA published a “Guideline on the requirements for quality documentation concerning biological investigational medicinal products in clinical trials“, providing important guidance on the structure and data requirements for a clinical trial application for exploratory and confirmatory trials of an ATMP. Here’s a checklist of the best practices that you should consider before submitting your ATMP for IMPD/IND approval:
About Cell Easy
Cell-Easy is a science-driven CDMO, specializing in advanced cell therapies. Cell easy provides a wide range of services, including process and analytical development, GMP cell banking, GMP manufacturing, and comprehensive CMC/regulatory support for global biotech and pharma companies. Our scientific team, combined with a Quality by Design (QbD) approach, ensures seamless technology transfer and development of cell-based therapies in oncology, autoimmunity, and regenerative medicine. This includes T cells, NK cells, MSCs, macrophages, HSCs, and exosomes.
Explore our services at www.cell-easy.com or contact us at info@cell-easy.com.