Our strength: understanding the Cell !
CELL BANKING
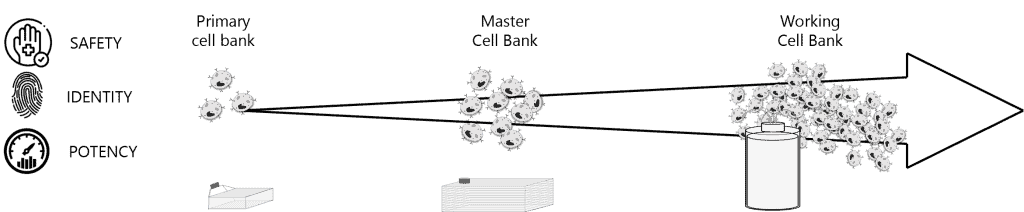
Cell Banking is a critical step in the development of biotherapeutics, ensuring the stable and consistent production of your biopharmaceuticals. It involves the creation and storage of Primary (PCB), Master (MCB) and Working Cell Banks (WCB) under strict ICH guidelines. These cell banks serve as the foundation for large-scale bioproduction, guaranteeing product consistency, safety, and efficacy throughout the manufacturing process.
What we can do for you
- Process Diagnostic and Familiarization: We initiate the process with an in-depth consultation to understand your project’s goals and outline a tailored cell banking strategy while embarking all your cell line specificities.
- Cell Bank Generation and QC testing: Using validated protocols, we develop the PCB and generate MCB and WCB while continuously monitoring stringent Quality Control. From 1mL to 50L our teams operate in state-of-the-art facilities (R&D and GMP) ensuring the stability and consistency of your cell lines.
30+
Cell banking projects

20+
in-house analytical assays

17
EU and US companies provided
- Fill & Finish and Storage: Up to 500 pharmaceutic vials, we offer tailored F&F strategy optimizing exposure time to toxic cryopreservation solution and controlled freezing program before long-time gazeous Nitrogen cryostorage.
- Documentation: All aspects of the GMP Cell Banking process are meticulously documented, providing a transparent trail of compliance with international regulatory guidelines.
ATMP MANUFACTURING
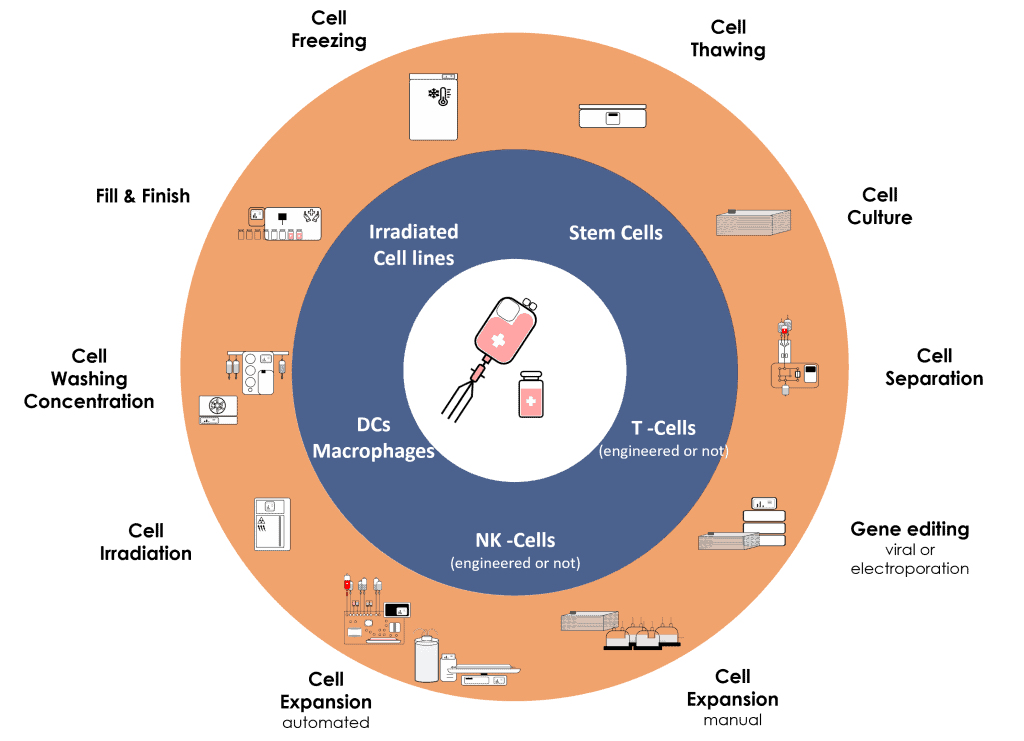
In pharmaceutical industry, the field of Cell Therapy is certainly the most complex in terms of both regulatory requirements and “Chemistry, Manufacturing, and Controls” (CMC).
Cell-based Products are living Drugs.
That’s why it’s crucial to fully understand all aspects of the clinical development of ATMPs from start to finish.
At Cell-Easy, our multi-disciplinary teams focus on SCIENCE and TECHNOLOGIES to propose solutions that meet product specifications and regulatory standards.
What we can do for you
- Controling the SUPPLY chain: Through partnerships with French Hospital/Clinic network and global Providers, we offer an extensive array of clinical-grade materials to support your clinical development.
- Developing Manufacturing Process & Analytical: From UpStream to DownStream Process Development, our tailored approach ensures robustness, cost-control, and time-savings to your process.
Our SCIENTIFIC approach combined with our REGULATORY expertise maximizes the potential for a seamless scale-up and GMP transfer.
GMP Manufacturing: Our experienced Production and QC Teams operate daily in a state-of-the-art facility authorized by the National French regulatory Agency (ANSM). From Technology Transfer to GMP Manufacturing, we support your projects with transparency and collaboration to find a solution to every manufacturing challenge.

20+
GMP clinical batches released

70+
Instruments & Technologies used

10+
IND submission
- Helping for QC and Regulatory: Our highly skilled Quality Assurance (QA) team, backed by a robust Quality Management System (QMS), has all the required procedures for Cell banking, Drug Substance batch release, and Drug Product batch certification for clinical use.
Combined with stringent QC procedures, you will obtain a complete control and traceability throughout the Manufacturing and Release of your Cell-based product.
- Facilitating access to patients: Sharing your passion for bringing advanced therapies to clinic, we have invested in cultivating relationships with over 10 local hospitals, facilitating streamlined access to 10,000+ patients.
This commitment is not only vital for the success of your programs but also for the well-being and success of the patients themselves.
MSC EXPERT
Mesenchymal Stem Cells (MSCs) hold great promise as a versatile tool in cell therapy, offering solutions for various medical conditions through their regenerative, immunomodulatory, and tissue-repairing properties.
Beyond variability brought by their sources (bone marrow, adipose tissue, umbilical cord,…), the large-scale manufacturing process itself leads variations in the IDENTITY and POTENCY of the generated cells.
At Cell-Easy, we’re committed to advancing allogeneic manufacturing processes for adipose-derived MSC, ensuring the generation of fully characterized, functional cells. Our Research and Clinical grade options cater to diverse activities, offering accessible solutions for various needs.
What we can do for you
- Providing adipose-derived MSC vials (RUO and GMP): Following our internal manufacturing/QC strategy, get a rapid access to ready-to-use MSC.
- Donor characterization.
- Pre-clinical datas on mouse model (toxicity, tumorogenicity).
- Analytical charecterization: Safety, Identity, Potency.
- Injected in First-in-Human studies through local partnership.
- Manufacturing customized batch: Benefit from our expertise to quickly access industrial quantities of adipose-derived MSCs while enjoying the clinical quality of our cells. The need of high quantity of MSCs is correlated to their use in various domain as Drug Product (engineered or not), starting material, intermediate cell bank, cargos, exosomes factory, meatable industry…

>800.109
Cells per tissue donation

>3,5.109
Cells per batch

25
analytical parameters screened
- Optimizing & Scaling-up your own manufacturing process: Our experienced Development Team is ready to provide knowledge and know-how on the MSC field and quickly find solutions to optimize and scale-up any MSC manufacturing process.
- Facilitating access to patients: Today, more than 10 clinicians are committed with Cell-Easy to inject adipose-derived MSCs to treat various diseases (severe systemic Sclerosis, Alzheimer disease, Crohn disease, bone fracture, critical limb Ischemia)
CMC & REGULATORY SUPPORT
Although Cell & Gene Therapy holds significant medical promise, it still grapples with immaturity across various fronts. Complex manufacturing processes, often unsuitable for industrial scale-up, coupled with lengthy and diverse regulatory pathways across different countries, present substantial hurdles.
The increasing complexity of manufacturing processes and regulations in the pharmaceutical and biotechnology industries has led to the creation of the CMC (Chemistry, Manufacturing, and Controls) profession.
Our seasoned expertise, streamlined teamwork, and science-driven approaches effectively minimize costs, enabling us to offer our services more competitively while passing on the benefits directly to you.
What we can do for you
- Program Diagnostic: Whether you’re a Start-up, Biotech company or Investor in the field of cell therapy, our track record in cell-based product development and manufacuring guarantees a thorough evaluation of your program, tailored to your company’s strategic objectives.
- CMC engineering and Technical support: We offer comprehensive technical assistance to clients equipped with in-house laboratories, extending guidance across both upstream and downstream processes to ensure seamless tech transfer and optimal outcomes.
- Quality and Regulatory guidance: Crafting a strategic regulatory approach that harmonizes a company’s business objectives, resources, and risk tolernce stands as a pivotal endeavor in product development. We meet your CMC quality and regulatory needs by designing and implementing quality systems tailored to your product stage. We also review/prepare all the required documentation (SOPs, qualification protocols, Batch Records) and efficiently cover all aspects of cGMP compliance to identify issues and propose appropriate corrective actions. Finally, we help preparing regulatory submissions including IND and can assist in meetings with your regulatory agency.
- Risk management _ de-risk your Budget: Leverage our hands-on expertise in risk management and analysis tools to simplify complexities and harness the power of this approach for:
- Identifying critical quality attributes (CQAs) for your product.
- Establishing precise product specifications.
- Prioritizing development studies and validation scope.
- Supporting ongoing investigations, such as manufacturing issues or product complaints.
- Managing materials, laboratory controls, stability studies, facility/equipment/utilities, and process characterization effectively.
- Project Management _ de-risk your Timelines: We provide extensive project planning and management support to ensure your development and clinical projects stay on track and within budget. Our team assists in integrating all your product development needs into comprehensive documentation, offering a seamless roadmap from product identification through clinical trials phases.