Our commitment: understanding our Customer !
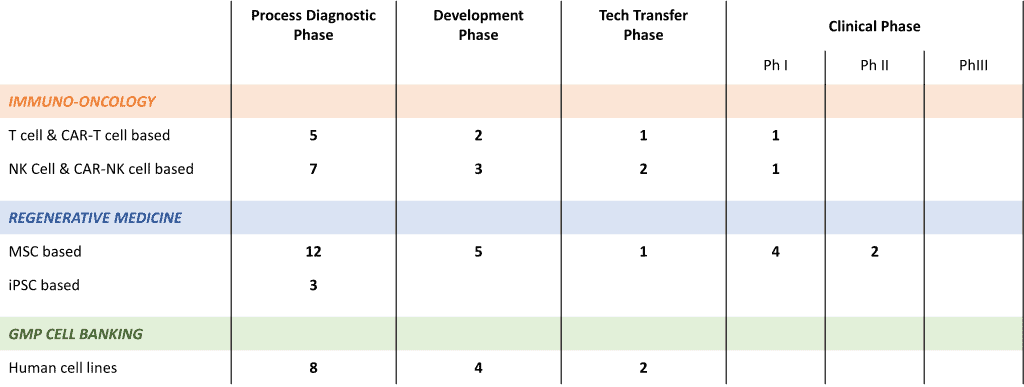
ATMP TRACK RECORD
Unlike all other therapeutic products, cell-based products are considerably more complex to manufacture.
In Cell Therapy, the PRODUCT is the PROCESS and the PROCESS is the PRODUCT !
It is essential to master the art of aseptic manufacturing as ATMPs cannot undergo terminal sterilization.
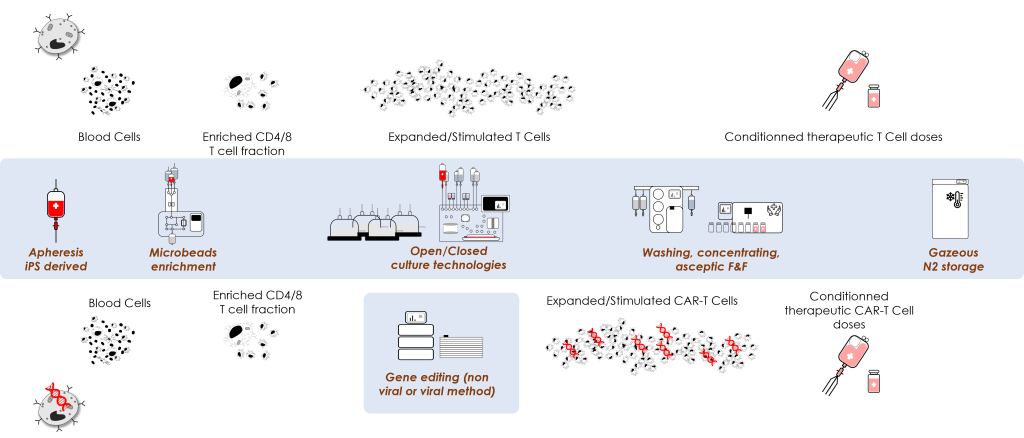
T Cells and CAR-T Cells
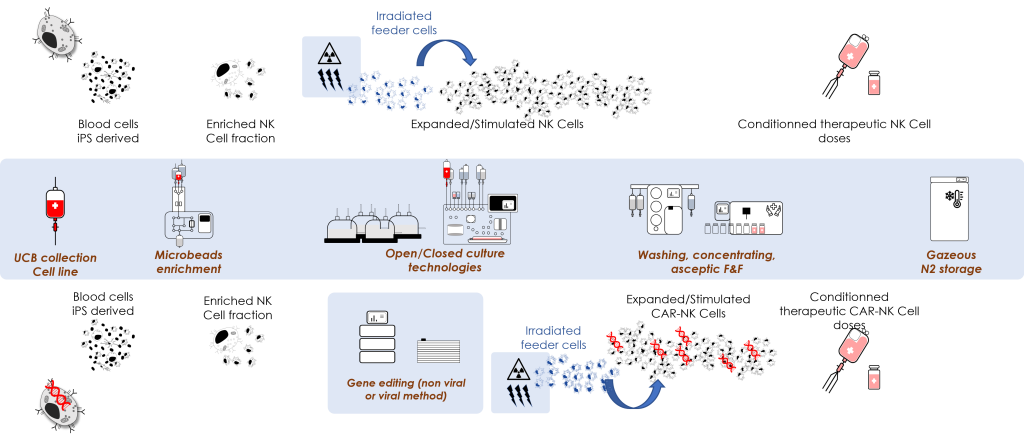
NK Cells and CAR-NK Cells
Mesenchymal Stem Cells
CASE STUDIES
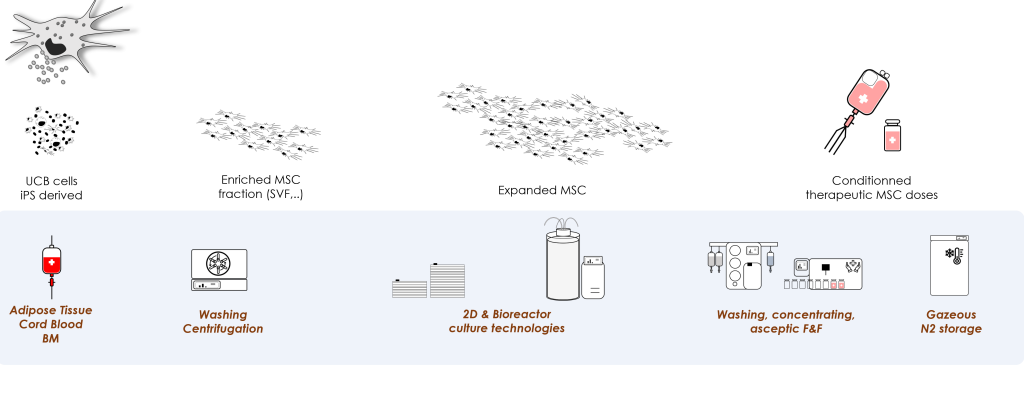
An off-the-shelf MSC product derived from lab-scale autologous process
- Problem Statement: Drawing on their scientific and medical expertise in the field of autologous MSCs, local researchers, professors and clinicians wanted to offer patients suffering from various autoimmune diseases an affordable and safe treatment.
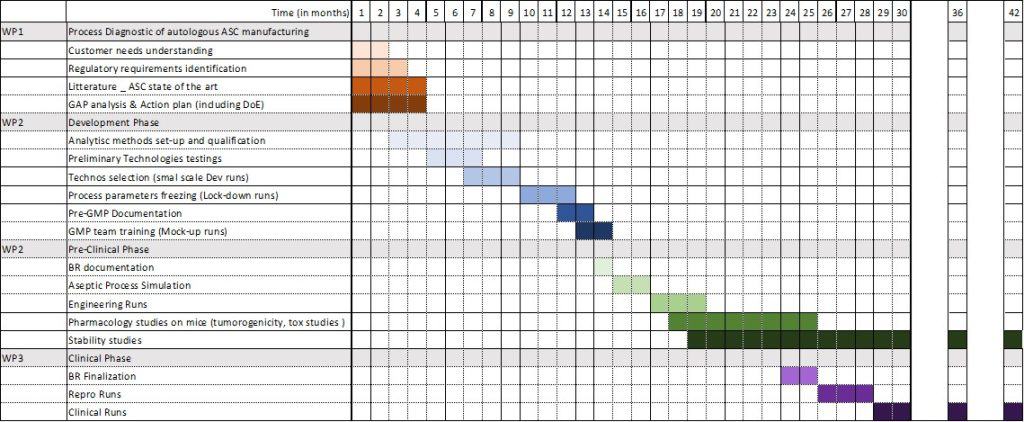
- Our Solution: In just 30 months, our dedicated teams have accomplished a significant milestone: the successful development, manufacturing, and validation of clinical-grade adipose-derived Mesenchymal Stem Cells (MSCs) based on a large-scale allogeneic process. These cells have received authorization from the French National Agency for Medicinal Products (ANSM) and are being injected in a range of Human clinical studies.
- Outcomes:
Cell-Easy is offering R&D and GMP grade adipose-derived MSCs as:
Study model for Research Programs
- Drug Product in various Clinical Trials (systemic sclerosis, Crohn disease, Alzheimer disease, arthritis diseases,…)
- Starting material for the generation of GMP exosomes
- Cargo for various molecules and viruses
Development of a non-proliferation test as release parameter

Cell Banking: Process scale-up

ALLIANCE
” ALONE we go faster, TOGETHER we go further ! “
The challenges are obviously technical and scientific, but they are definitely medico-economic. Therefore, developing cell-based therapies involve mastering technical, scientific, industrial manufacturing and regulatory constraints.
Mutualization of skills is essential in a field where the complexity of the issues is predominant.
This translates among other things into:
- One clear, detailed and unambiguous proposal
- A Technology Transfer driven by Communication, Co-working and Go/NoGo de-risking approach
- A constant flexibility in integrating unforeseen complementary studies, without necessarily contracting “Change of scope” Proposal
- A risk sharing assessment to ensure successful target achievement while respecting initial budget.