Our pioneer Mesenchymal Stem Cells complete solution: from bench to bedside !
MSC4all Platform : the Origin
Thanks to our scientific knowledge of stem cells, we have successfully refined an R&D process into a clinical-ready cell therapy product. Our combined Cellprocess & Cellanalytics Platforms have been instrumental to achieve this. As a result, we have converted an autologous process into an allogenic one, expanding manufacturing capabilities, reducing costs, and enhancing cell characterization.
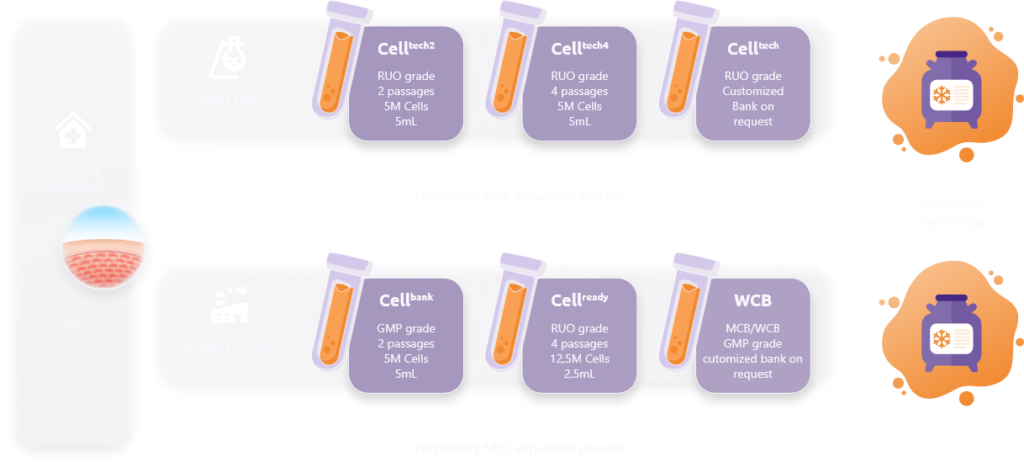
We offer today MSCs from RUO grade (Research Use Only) to GMP clinical grade, along with a full offering of Primary Cell Bank (SVF=Stromal Vascular Fraction), Master Cell Bank (CellBank), Working Cell Bank (CellReady).
250.109
Cells from 1
Adipose tissue donation
>3.109
Cells per Batch
x10
cost reduction
As an MSC-based Drug Developer, you need a cost-efficient manufacturing process. As an MSC-expert CDMO player, we provide full-qualified MSC Products including customized regulatory assistance from cell sourcing to dossier authoring services.
The complete MSC portfolio
Contact
Tel : +33 534 276 550
Address : Cell-Easy SAS, 4 bis avenue Hubert Curien, 31100 Toulouse, France
Mail : info@cell-easy.com
Bridging the gap between R&D Project and clinical Development