De-Risk & Accelerate Your Journey To The Clinic With Your Cell Therapy CDMO Partner
We support academic laboratories, biotechs and pharma companies through GMP and regulatory challenges, helping to reduce risk, speed up timelines, and build strong, trusted collaborations so you can focus on what matters: advancing your therapy with clarity and peace of mind.
1000+
Clinical doses
15
Cell Types
50+
Development Programs
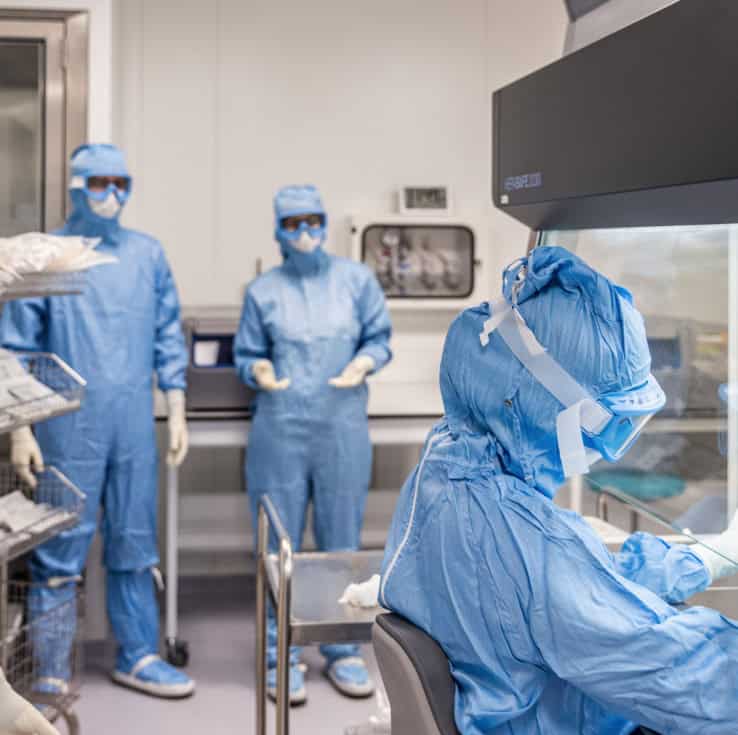
Cell-Based Therapies Are Not Conventional Drugs. They’re Living Treatments
Whether you’re preparing for a first-in-human trial or scaling up for late-phase manufacturing, success requires more than GMP compliance. It demands scientific excellence in cell culture, early and continuous risk anticipation, and deep regulatory insight at every stage. With fewer than 1% of cell therapies reaching the market, the stakes are high. Every decision, from process design to CMC strategy, can impact patient safety, regulatory approval, and commercial viability. That’s why our clients partner with us across all stages of development: to de-risk, accelerate, and succeed.
CDMO Services To Accelerate Your Cell Therapy Development
Process Diagnostic
& Development
Analytical Development
cGMP Cell Banking
cGMP Manufacturing
Case Study: Solving the Scale-Up Barrier: How We Engineered a High-Yield, In Vivo-Ready T-Cell Process
Scaling up a T-cell therapy from lab-scale to large-scale manufacturing is challenging. Traditional T-flask methods consume excessive resources, require extensive manual work, and increase contamination risks. Our goal was to transform a small-scale proof-of-concept into a robust, scalable process suitable for in vivo studies.

Latest news
Stay on top of our latest breakthroughs.
CHU Toulouse and Cell-Easy Receive Regulatory Approval of A3D: A Phase 1 Trial Using CellReady® in Alzheimer’s Disease
Learn more: CHU Toulouse and Cell-Easy Receive Regulatory Approval of A3D: A Phase 1 Trial Using CellReady® in Alzheimer’s DiseaseCHU Toulouse and Cell-Easy Receive Regulatory Approval of A3D: A Phase 1 Trial Using CellReady® in Alzheimer’s Disease Cell-Easy, a

Cell-Easy Expands its GMP Cell Therapy Manufacturing Facility to Support Growing Client Demand
Learn more: Cell-Easy Expands its GMP Cell Therapy Manufacturing Facility to Support Growing Client DemandCell-Easy Expands its GMP Cell Therapy Manufacturing Facility to Support Growing Client Demand New state-of-the-art class-B cleanrooms, development labs, and

CTA Approval to initiate ALLOFIST
Learn more: CTA Approval to initiate ALLOFISTCTA Approval to initiate ALLOFIST CHU Toulouse receives CTA Approval to initiate ALLOFIST, a Phase 1/2 Trial based on Cell-Easy’s
Let’s talk about your project
Let’s turn your vision into a reality.
Phone: +33 534 276 550
Address: Cell-Easy SAS, 4 bis avenue Hubert Curien,
31100 Toulouse, France
Mail: info@cell-easy.com






