The CDMO Expert
in Cell Therapy
Products
Cell-based therapies are not conventional Biologics,
they are living drugs…
…requiring cutting-edge expertise in Cell Culture.
Clinical Doses
manufactured in our state-of-the-art GMP facilities

Cell types
T cells, NK cells, T regs, MSCs, iPS cells, Macrophages, immortalized cell lines,…

Development Programs
Process Development, Optimization, Scale up.
Analytical & Potency test development.

ATMP Developers
TRUST US
from academic, to Biotech and Pharma companies
How SCIENCE plays out at Cell-Easy?
By combining solid technological and scientific knowledge in CELL CULTURE with conventional regulatory-controlled CDMO services, Cell-Easy’s multi-disciplinary team support you in transforming your laboratory proof-of-concept into a successful first-in-human clinical trial.
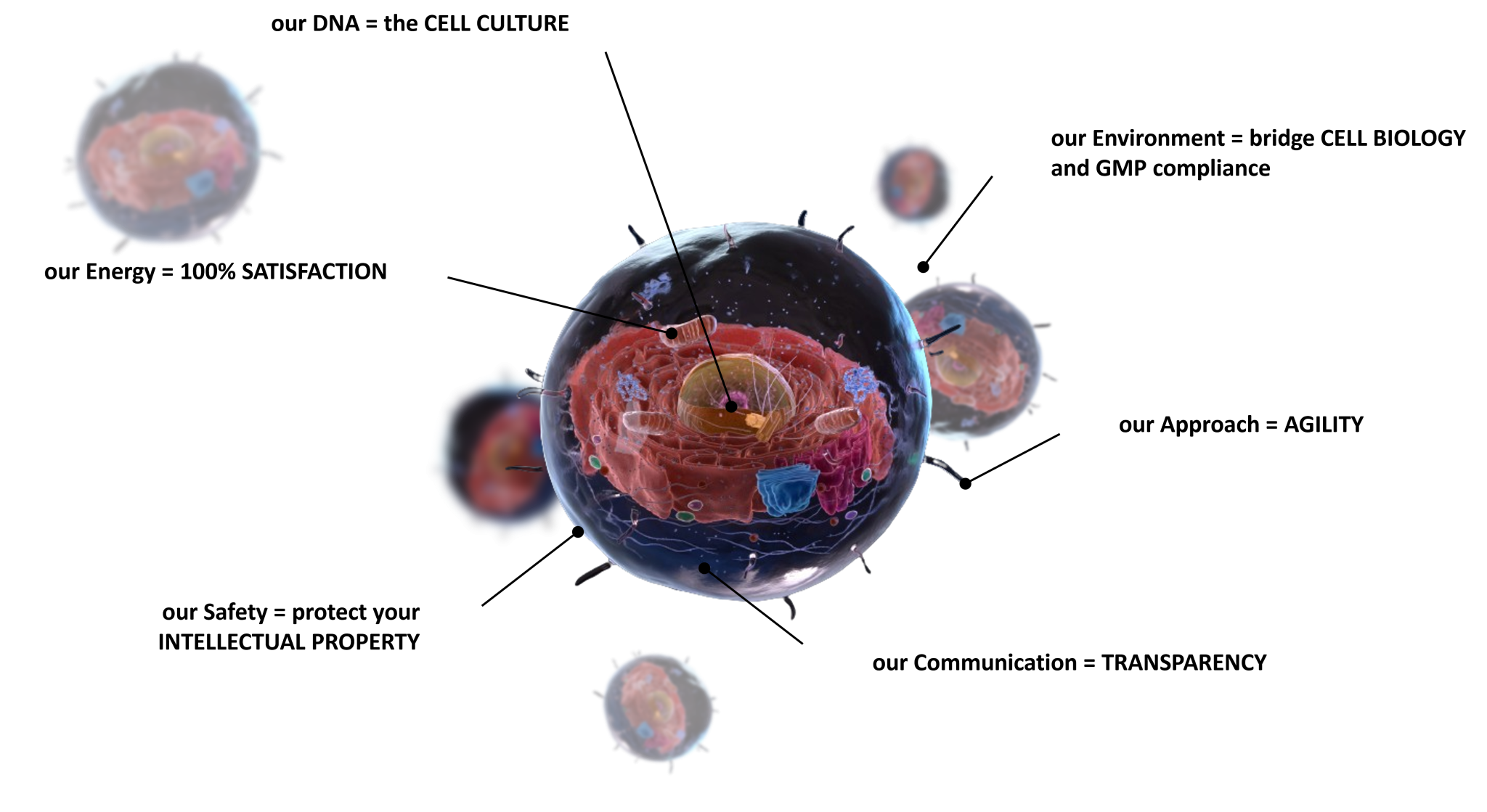
- Teams = PhDs, Engineers, Technicians in Cell Biology
- 70+ Technologies for Process & Analytics Development
- REGULATORY support adapted to cell products
- CMC driven by cell variability
- From concept to First-in-Human and beyond
- A dedicated Project Manager
- Co-define a robust roadmap
- Share Risks & Gaps during weekly meeting
- Avoid Change of Scope
- Accelerate Process Development/Scale-up with our Platforms
- Access expertise on 50+ different technologies
- Pre-established contract with providers
- Beyond agreements (NDA, MSA, QTA) our internal confidential policy protect your industrial secret
- As a fee for service CDMO, no IP is shared
- All documentation/production is Yours
- Respect TIMELINES
- Meet BUDGET
- Bring continous CMC and REGULATORY support
- Your SUCCESS is our goal

Cell-Easy is pleased to welcome Mrs. Gisele Deblandre, formerly CSO at MaSTherCell, as Chief Scientific Officer.
Toulouse, France, September 21st of 2023 Cell-Easy, a specialized contract development and manufacturing organization (CDMO) in cell therapy, proudly announces the appointment of Mrs. Gisele Deblandre as the Chief Scientific Officer. Over the years, Cell-Easy has recognized the challenges faced by biotechs in bridging the gap between early-stage projects

IUCT-Oncopole and Cell-Easy sign a MOU to accelerate cancer patient access to advanced cell-based therapy
TOULOUSE, FRANCE, June 22, 2023 IUCT-Oncopole and Cell-Easy SAS (Cell-Easy) announced today the signing of a Memorandum of Understanding to enhance their strategic collaborative relationship and open access to advanced cell-based therapies. This partnership aims to drive innovation in public health and improve access to promising new cancer therapies for patients. By

Cell-Easy Appoints CDMO Expert Dr. Ribault to Its Board
TOULOUSE, France _ April 10, 2023 Cell-Easy is a leading contract development and manufacturing organization (CDMO) with a strong foothold in Good Manufacturing Practice (GMP) manufacturing services for immune cells (T, NK cells) and adult stem cells (MSCs, iPSCs). Since 2020, the company keeps expanding into genetically modified cells

EMERCell & Cell-Easy sign a Strategic Agreement for the Scale-up and Manufacturing of NK-001
TOULOUSE, France & MONTPELLIER, France _ September 22, 2022 Emercell has selected Cell-Easy as a long-term CDMO partner for the scale-up and manufacturing operations of its allogeneic NK cell-based product, with the objective to enter clinical phase in 2023. Emercell has signed a strategic agreement with Cell-Easy specialized in the
They Trust Us
Contact
Tel : +33 534 276 550
Address : Cell-Easy SAS, 4 bis avenue Hubert Curien, 31100 Toulouse, France
Mail : info@cell-easy.com









