The CDMO Expert
in Cell Therapy
Products
Cell-based therapies are not conventional Biologics,
they are living drugs…
…requiring cutting-edge expertise in Cell Culture.
Clinical Doses
manufactured in our state-of-the-art GMP facilities

Cell types
T cells, NK cells, T regs, MSCs, iPS cells, Macrophages, immortalized cell lines,…

Development Programs
Process Development, Optimization, Scale up.
Analytical & Potency test development.

ATMP Developers
TRUST US
from academic, to Biotech and Pharma companies
How SCIENCE plays out at Cell-Easy?
By combining solid technological and scientific knowledge in CELL CULTURE with conventional regulatory-controlled CDMO services, Cell-Easy’s multi-disciplinary team support you in transforming your laboratory proof-of-concept into a successful first-in-human clinical trial.
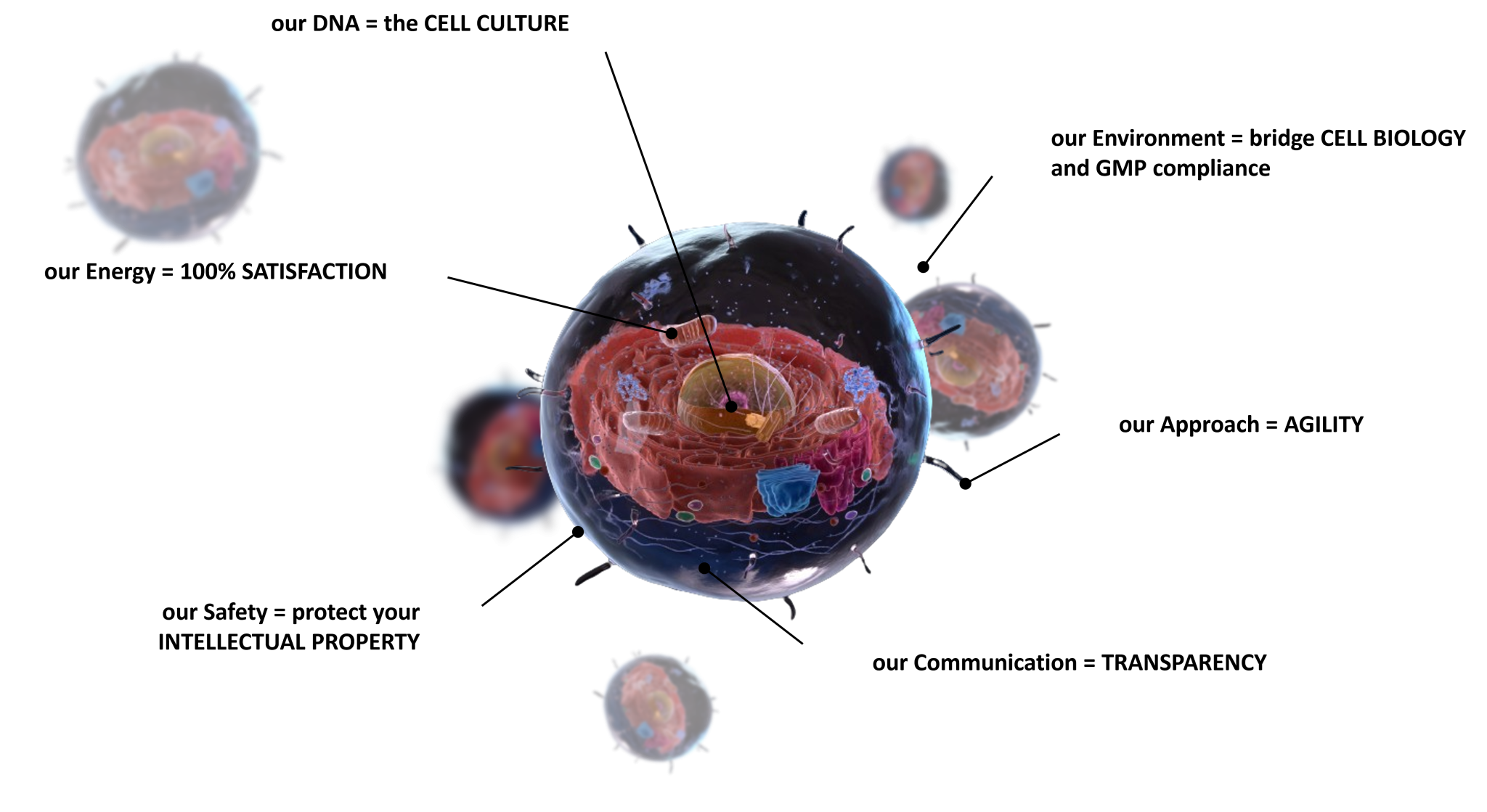
- Teams = PhDs, Engineers, Technicians in Cell Biology
- 70+ Technologies for Process & Analytics Development
- REGULATORY support adapted to cell products
- CMC driven by cell variability
- From concept to First-in-Human and beyond
- A dedicated Project Manager
- Co-define a robust roadmap
- Share Risks & Gaps during weekly meeting
- Avoid Change of Scope
- Accelerate Process Development/Scale-up with our Platforms
- Access expertise on 50+ different technologies
- Pre-established contract with providers
- Beyond agreements (NDA, MSA, QTA) our internal confidential policy protect your industrial secret
- As a fee for service CDMO, no IP is shared
- All documentation/production is Yours
- Respect TIMELINES
- Meet BUDGET
- Bring continous CMC and REGULATORY support
- Your SUCCESS is our goal
News
Accelerating Patient Access to Advanced Cell-based Therapies through the Signature of a Service Agreement between the French CDMO and the Toulouse University Hospital (CHU Toulouse).
Accelerating Patient Access to Advanced Cell-based Therapies through the Signature of a Service Agreement between the French CDMO and the Toulouse University Hospital (CHU Toulouse) Toulouse, France, April 18, 2024 – This agreement between the two parties opens great perspectives for Biotechs, providing them with seamless access to patients who
Cell-Easy Signs Groundbreaking Manufacturing Contract, Paving the Way for Local Hospitals to Offer Next-Generation CAR-T Therapy.
The accumulated expertise among various programs as well as our local partnerships have been the foundation to close such a deal. I’m a true believer that our positioning can be beneficial for other biotech seeking to easily access patient cohorts. Alexis Delbaere Tweet About Cell-Easy Cell-Easy is a science-centric CDMO

IMPD Approval for FIH Trial in Systemic Sclerosis – Cell Easy
EU regulatory approval to administer Adipose-derived mesenchymal Stem Cells (ASCs) in patients with ischemic digital ulcers marks key milestone for partners Toulouse, France, November 16, 2023 – Cell-Easy, a French Contract Development and Manufacturing Organization (CDMO) focused on advanced cell therapies, and the Toulouse University Hospital, today announce that they

IUCT-Oncopole & Cell-Easy Join Forces in Cell Therapy
TOULOUSE, FRANCE, September 19th, 2023 Cell-Easy, a specialized contract development and manufacturing organization (CDMO) in cell therapy, proudly announces the appointment of Mrs. Gisele Deblandre as the Chief Scientific Officer. Over the years, Cell-Easy has recognized the challenges faced by biotechs in bridging the gap between early-stage projects and clinical
They Trust Us
Contact
Tel : +33 534 276 550
Address : Cell-Easy SAS, 4 bis avenue Hubert Curien, 31100 Toulouse, France
Mail : info@cell-easy.com








